half life formula for first order reaction
The half-life of a chemical reaction is the time required for the concentration of the reactants to reach half of their initial value. Example of First Order Reaction.

Reaction Rates And Kinetics Notes Chemistry Textbook Study Chemistry Chemistry Notes
Thus the half-life of a second-order reaction unlike the half-life for a first-order reaction does depend upon the initial concentration of A.

. Also the large box I have only holds 9 cartons so I have quite a supply red cooler bags we use them for just about everything A few times over the last year weve gotten maybe 10 cartons that were a little chunky. As the initial concentration of A increases the half-life decreases. Half gallons are easy to store in the fridge but with 3 boys the 9 cartons were a little annoying to bring in.
The reason the 1st order reaction gives a half-life is that when the equation integrates it produces a logarithmic function that produces a quotient when subtracted and the starting quantities cancel by division. For first-order reactions the relationship between the reaction half-life and the reaction rate constant is given by the expression. Specifically there is an inversely proportional relationship between latextextt_frac12latex and A 0.
Consider the hydrolysis of ethyl acetate during the hydrolysis the concentration of ethyl acetate is 002 molL whereas the amount of water is 20 molL as the process of hydrolysis involves a large amount of waterLet us say the process of hydrolysis attains completion in. Here is an example to help you understand the concept more clearly. A B and C are altered only marginally so you have a pseudo-1st-order reaction which then has a definable half-life.

Pin On Organic Chemistry Notes

Chemical Kinetics In 2021 Chemical Kinetics Chemistry Experiments Chemistry Class 12

Concentration Units Formulae Analytical Chemistry Examples Urdu Hin Chemistry The Unit Concentration

Radioactive Isotopes Of Lead Radioactive Secondary Science Radioactive Iodine

Chemical Equilibrium Reaction Online College Chemistry Courses College Chemistry Chemical Kinetics Online Degree

Concentration Units Formulae Analytical Chemistry Examples Urdu Hin Chemistry The Unit Concentration

Ncert Solutions For Class 12 Chemistry Chapter 4 Chemical Kinetics Cbse Tuts Chemicalkineticsclass12ncertsolutions Chemical Kinetics Chemistry Solutions

What Is Second Order Of Kinetics Kinetics Ppt Chemistry Classroom Chemistry Basics Chemical Kinetics

Fig I 1 Change Of The Rate Of Hydrolysis Of Ethyl Acetate With Temperature As Temperature Increases By 10 K Chemical Kinetics Chemical Reactions Ap Chemistry

Electrochemistry Notes Chemistry Notes Electrochemistry Chemistry Lessons

Pin By Lily Sue On Stem Physical Science Chemistry Classroom Chemistry Lessons Chemistry Education

Zero Order Kinetics In 2021 Chemistry Textbook Chemistry Classroom Chemistry Experiments

Alum Formula Chemistry Chemistry Math Formula

Organic Chemistry Notes Full Course Pdf Notes Chemistrynotes Com Chemistry Notes Organic Chemistry Notes Organic Chemistry Study

Chemical Kinetics And Half Life Online College Chemistry Courses Chemical Kinetics Half Life Physical Chemistry

Ncert Solutions For Class 11 Chemistry Chapter 8 Redox Reactions 025 Redox Reactions 11th Chemistry Chemistry
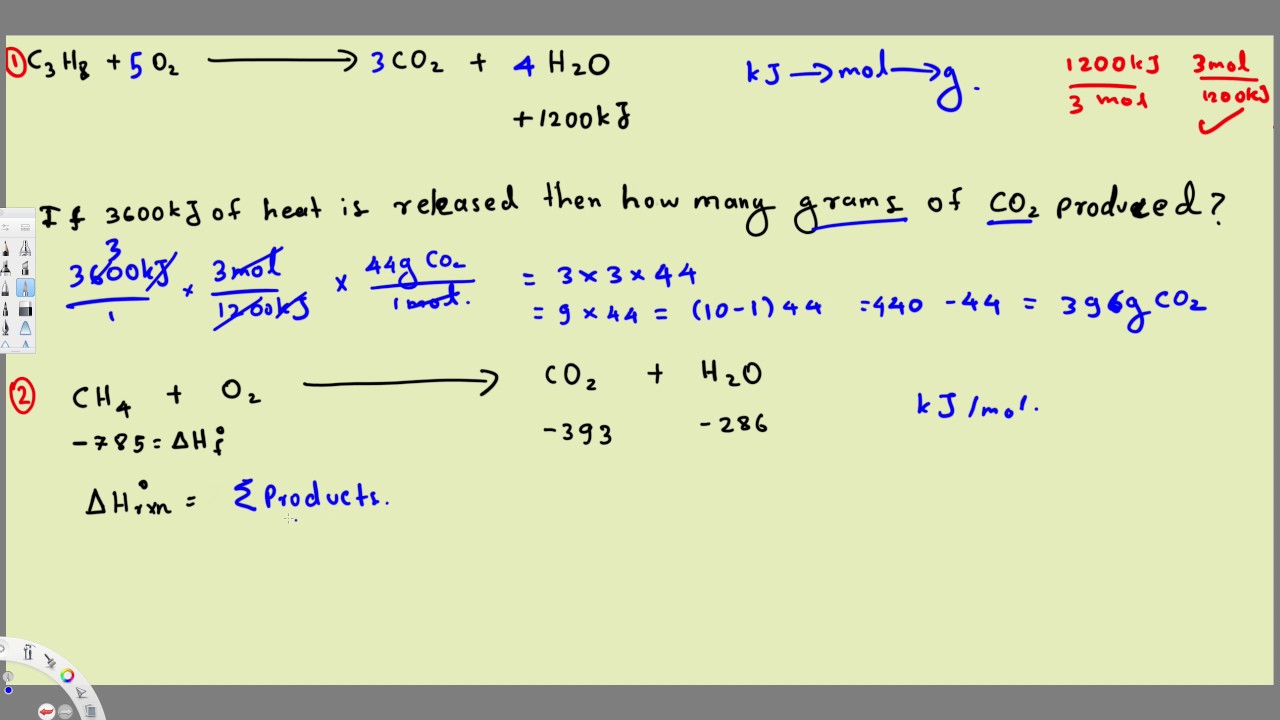
Thermochemistry Equations Formulas Practice Problems Example 2 Equations Chemistry Practice

Statistics Chapter Wise Important Questions Class 10 Mathematics Cbse Tuts Statisticsclass9extraquestions Math Mathematics Chapter

Practical Centre Introduction To Chemical Kinetics Mcqs Chemistry Xi Chemical Kinetics Chemistry Chemistry Notes